Advancing mRNA drug development
How Poly-A Tail Modification improve mRNA stability?
Articles were recently published in the TriLink BioTechnologies, part of Maravai LifeSciences describing groundbreaking work to improve mRNA stability and translation by introducing branched poly-A-tail into mRNAs using click chemistry. These modified mRNAs exhibited prolonged translation and stability, resulting in reduced immunogenicity. This development represents a significant leap forward in the field of mRNA drug discovery and offers new strategies to improve the efficiency and efficacy of mRNA-based therapies. The use of click chemistry to modify poly-A tails is a novel approach that could lead to more robust and versatile mRNA vaccines and therapeutics.
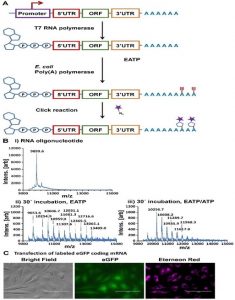
Trilink’s summary is based on two publications by Chen et al. from the Broad Institute and Massachusetts Institute of Technology, published in Nature Technology in May 2024[1], and by Adiham et al., published in 2022[2]. These fundamental studies pave the way for new methods to improve mRNA stability and translation efficiency, which are essentional for the development of mRNA-based therapeutics. At baseclick, we published the basis for these further advances in the modification of the poly-A tail of mRNA with a clickable alkyne group in Croce et al. in 2020[3] (Figure 1) The alkyne group can undergo click chemistry reaction without compromising the integrity of the mRNA (see Figure 1). This concept was further refined in the two new publications.
Figure 1. EATP labeling of the poly(A) tail. A) Schematic representation of EATP incorporation into the poly(A) tail of IVT mRNA. B) MALDI-TOF results from from E. coli poly(A) polymerase reactions after 30 min incubation using i) no nucleotide, ii) EATP and iii) EATP/ATP. C) Microscopy images of HeLa cells transfected with eGFP mRNA labeled with Eterneon Red fluorescent dye in poly(A) tail. Left to right: bright field, green fluorescence, and Cy5 channel. Scale bar: 100 mm.
These further advancements in poly-A tail stabilization will help to advance mRNA research within the RNA community. By sharing insights and breakthroughs, we can collectively push the boundaries of what’s possible in mRNA vaccine and drug development. We are thrilled to see such promising progress and are committed to continuing our efforts in developing innovative solutions to enhance mRNA translation. The future of mRNA-based therapies is brighter than ever, and we look forward to the new possibilities these advancements will unlock. For more information on our work and to read the full paper, visit our website or reach out to our team. Together, we can drive the future of mRNA technology forward.